Articles from Womed

Womed®, the uterine health company developing innovative intrauterine treatments to free women from uterine pathologies, today announced that the Food and Drug Administration approved the PreMarket Approval (PMA) application of the Womed Leaf® for adult women undergoing hysteroscopic surgery for symptomatic moderate to severe intrauterine adhesions, also referred to as Asherman syndrome. Womed Leaf® is the first medical device to be approved for sale in the United States for that indication.
By Womed · Via Business Wire · September 16, 2025
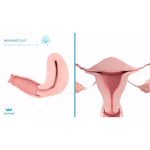
Womed, the women's health company pioneering innovative, safe and effective treatments to free women from uterine pathologies, today announced that the results of the PREG2 clinical trial demonstrated Womed Leaf® is effective in the management of severe and moderate intrauterine adhesions (IUAs), the primary mechanical cause for female infertility. No device indicated for IUA prevention has been shown to be effective in this specific patient group. The PREG2 trial results were presented today by Prof. Jean-Louis Benifla, head of the ObGyn department at Lariboisière Hospital – APHP, Paris, France, during the Choix des Armes conference in Marseille, France.
By Womed · Via Business Wire · March 7, 2024
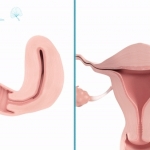
Womed, the women's health tech company pioneering innovative treatments against challenging uterine pathologies based on a novel polymer material technology, today announced the publication in the Journal of Minimally Invasive Gynecology (JMIG) of results of the clinical trial evaluating its first product, Womed Leaf™. The clinical data demonstrated that the device is safe and effective in preventing intrauterine adhesion in women with uterine fibroids undergoing hysteroscopic myomectomy. Womed also announced that Womed Leaf™ received CE Marking.
By Womed · Via Business Wire · September 8, 2021
