Articles from Avenacy

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it will be attending the 2025 American Society of Health-System Pharmacists (ASHP) Midyear Clinical Meeting and Exhibition in Las Vegas, Nevada from December 7-10, 2025. At the conference, the Avenacy leadership team will connect with existing and potential customers to share the progress the Company has made over the past two years. Interested parties can visit Avenacy at exhibit booth #1467.
By Avenacy · Via Business Wire · December 3, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced that it will be attending CPHI Frankfurt 2025, taking place October 28th-30th.
By Avenacy · Via Business Wire · October 22, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced the launch of their first Lidocaine Hydrochloride Injection, USP in the United States. The product is a generic equivalent of XYLOCAINE®, as approved by the U.S. Food and Drug Administration, and is indicated for the production of local or regional anesthesia or analgesia. Each vial of 1% Lidocaine HCl Injection, USP contains 500 mg per 50 mL (10 mg per mL) and is available as a carton of 25 multi-dose vials.
By Avenacy · Via Business Wire · August 25, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced the launch of Dehydrated Alcohol Injection, USP in the United States as a therapeutic generic equivalent of Ablysinol®, which ended its market exclusivity in June 2025.
By Avenacy · Via Business Wire · July 21, 2025

Avenacy, a specialty pharmaceutical company committed to supplying critical injectable medications to the U.S. market, today announced that it has entered into an Asset Based Lending (ABL) agreement with Texas Capital Bank. The agreement provides Avenacy with the financial flexibility to accelerate its growth and support the continued expansion of its product portfolio to better serve healthcare providers, hospitals, and patients nationwide.
By Avenacy · Via Business Wire · June 24, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Leuprolide Acetate Injection in the United States. The product is a therapeutic generic equivalent to LUPRON®, as approved by the U.S. Food and Drug Administration, and is indicated for the palliative treatment of advanced prostate cancer. Avenacy’s Leuprolide Acetate Injection is available as a 14-Day Patient Administration Kit, which includes one multi-dose vial containing 14 mg per 2.8 mL (1 mg per 0.2 mL), 14 disposable syringes, and 28 alcohol wipes.
By Avenacy · Via Business Wire · June 2, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Doxycycline for Injection, USP in the United States as a therapeutic generic equivalent for DOXY 100®, as approved by the U.S. Food and Drug Administration.
By Avenacy · Via Business Wire · May 27, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Propofol Injectable Emulsion, USP in the United States as a therapeutic generic equivalent for Diprivan® as approved by the U.S. Food and Drug Administration. Propofol Injectable Emulsion, USP is an intravenous general anesthetic and sedation drug indicated for:
By Avenacy · Via Business Wire · April 7, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched a suite of antibiotic products for injection, including:
By Avenacy · Via Business Wire · March 17, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced that it will be attending DCAT Week from March 17-20, 2025 in New York City.
By Avenacy · Via Business Wire · March 10, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Zoledronic Acid Injection, USP in the United States as a therapeutic generic equivalent for Zometa® as approved by the U.S. Food and Drug Administration. Zoledronic Acid Injection, USP is indicated for the treatment of hypercalcemia of malignancy and for the treatment of multiple myeloma and bone metastases of solid tumors, in conjunction with standard antineoplastic therapy.
By Avenacy · Via Business Wire · February 18, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today provided an update on recent business highlights and financial performance for 2024 and strategic priorities for 2025 ahead of the Company’s participation in the 43rd Annual J.P. Morgan Healthcare Conference from January 13-16, 2025 in San Francisco, CA.
By Avenacy · Via Business Wire · January 6, 2025

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced that it will be attending the 43rd Annual J.P. Morgan Healthcare Conference from January 13-16, 2025 in San Francisco, CA.
By Avenacy · Via Business Wire · December 16, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it will be attending the 2024 American Society of Health-System Pharmacists (ASHP) Midyear Clinical Meeting & Exhibition in New Orleans, Louisiana from December 8-12, 2024. At the conference, the Company will be showcasing its progress since launch, differentiated business model, and experienced leadership team, as well as hosting meetings with potential customers and partners as it expands its global presence and portfolio of critical injectable medicines. Interested parties can visit Avenacy at exhibit booth #1029.
By Avenacy · Via Business Wire · December 2, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Dexmedetomidine Injection, USP in the United States as a therapeutic generic equivalent for Precedex® as approved by the U.S. Food and Drug Administration. Dexmedetomidine Injection, USP is an alpha2-adrenergic receptor agonist indicated for:
By Avenacy · Via Business Wire · November 18, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical medications, looks forward to celebrating its first year of growth and success since launching in October 2023. Backed by a robust global network of development and FDA-inspected contract manufacturing partners, Avenacy has already brought 13 injectable products to the U.S. market to address key gaps in the drug supply chain.
By Avenacy · Via Business Wire · September 23, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Metoclopramide Injection, USP in the United States as a therapeutic generic equivalent for Reglan® as approved by the U.S. Food and Drug Administration. Metoclopramide Injection, USP is indicated for the relief of symptoms associated with diabetic gastroparesis, the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, the prevention of postoperative nausea and vomiting, small bowel intubation, and radiological examination.
By Avenacy · Via Business Wire · September 16, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced Dr. Patrick Soon-Shiong, a pharmaceutical industry veteran, businessman, and investor, will join as co-founder and board member. Funding from NantCapital will enable execution of Avenacy’s long-term strategy and support the Company’s next phase of growth, as it prepares to launch additional essential injectable medications and scale the business to meet growing demand for its portfolio of critical medicines.
By Avenacy · Via Business Wire · August 20, 2024
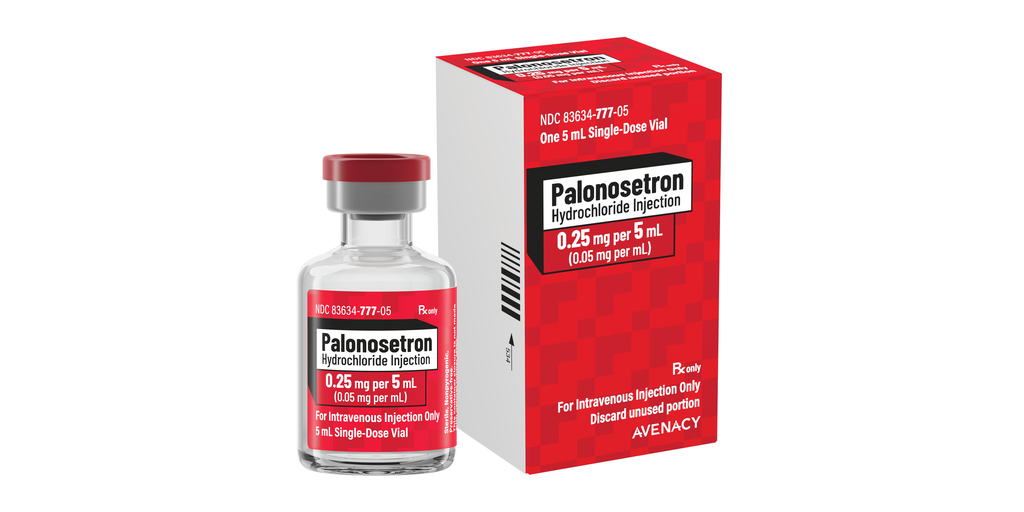
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Palonosetron Hydrochloride Injection, USP in the United States as a therapeutic generic equivalent for Aloxi® as approved by the U.S. Food and Drug Administration. Palonosetron Hydrochloride Injection, USP is indicated as an antiemetic.
By Avenacy · Via Business Wire · July 22, 2024
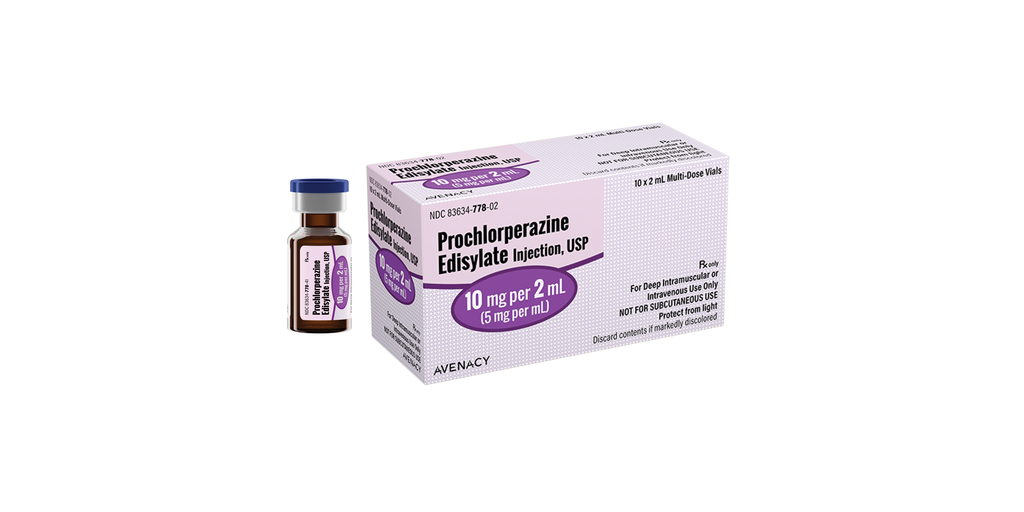
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Prochlorperazine Edisylate Injection, USP in the United States as a therapeutic generic equivalent for COMPAZINE® as approved by the U.S. Food and Drug Administration. Prochlorperazine Edisylate Injection, USP is indicated to control severe nausea and vomiting and for the treatment of schizophrenia. Prochlorperazine has not been shown effective in the management of behavioral complications in patients with mental retardation.
By Avenacy · Via Business Wire · June 26, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Tranexamic Acid Injection, USP in the United States as a therapeutic generic equivalent for Cyklokapron® as approved by the U.S. Food and Drug Administration. Tranexamic Acid Injection, USP is indicated in patients with hemophilia for short-term use (2 to 8 days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.
By Avenacy · Via Business Wire · June 24, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Isoproterenol Hydrochloride Injection, USP in the United States as a therapeutic generic equivalent for ISUPREL® as approved by the U.S. Food and Drug Administration. Isoproterenol Hydrochloride Injection, USP is indicated:
By Avenacy · Via Business Wire · May 20, 2024
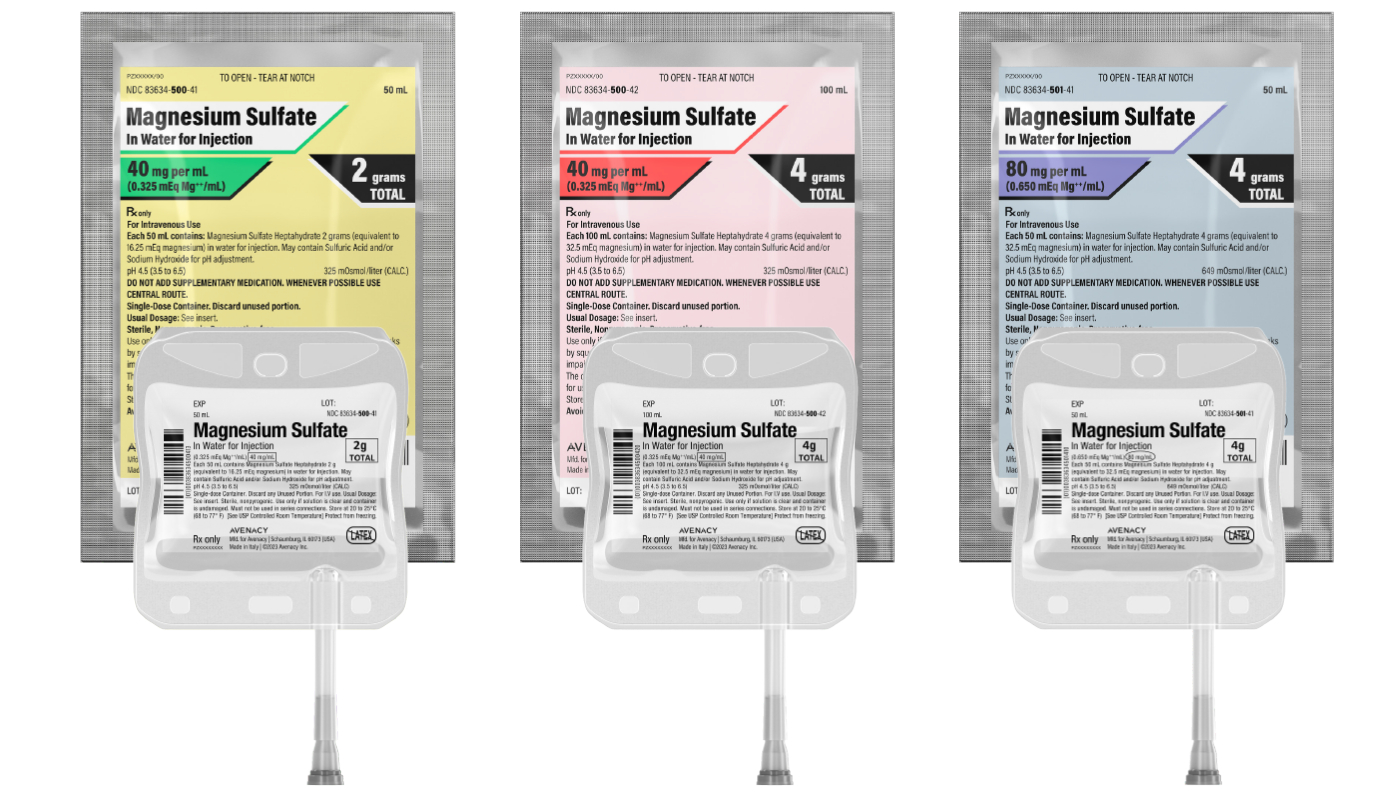
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Magnesium Sulfate in Water for Injection in the United States as approved by the U.S. Food and Drug Administration. Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively. When used judiciously, it effectively prevents and controls the convulsions of eclampsia without producing deleterious depression of the central nervous system of the mother or infant. However, other effective drugs are available for this purpose.
By Avenacy · Via Business Wire · May 14, 2024
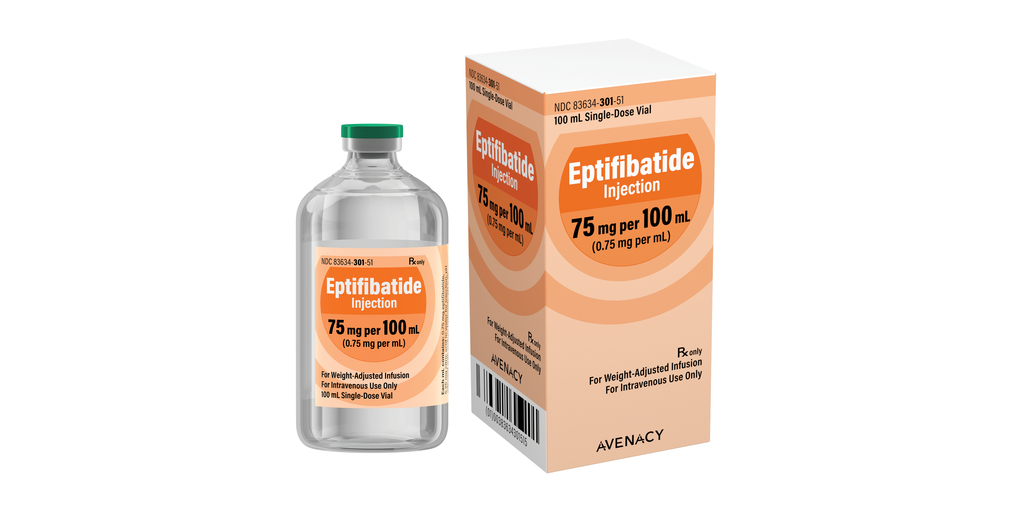
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Eptifibatide for Injection in the United States as a therapeutic equivalent generic for Integrilin® for Injection (Eptifibatide) approved by the U.S. Food and Drug Administration. Eptifibatide for Injection is indicated for use in acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI).
By Avenacy · Via Business Wire · April 29, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Desmopressin Acetate for Injection in the United States as a therapeutic equivalent generic for DDAVP® for Injection (Desmopressin Acetate) approved by the U.S. Food and Drug Administration. Desmopressin Acetate for Injection is multi-indicated for patients with central diabetes insipidus, hemophilia A, and von Willebrand’s disease (Type I).
By Avenacy · Via Business Wire · April 15, 2024
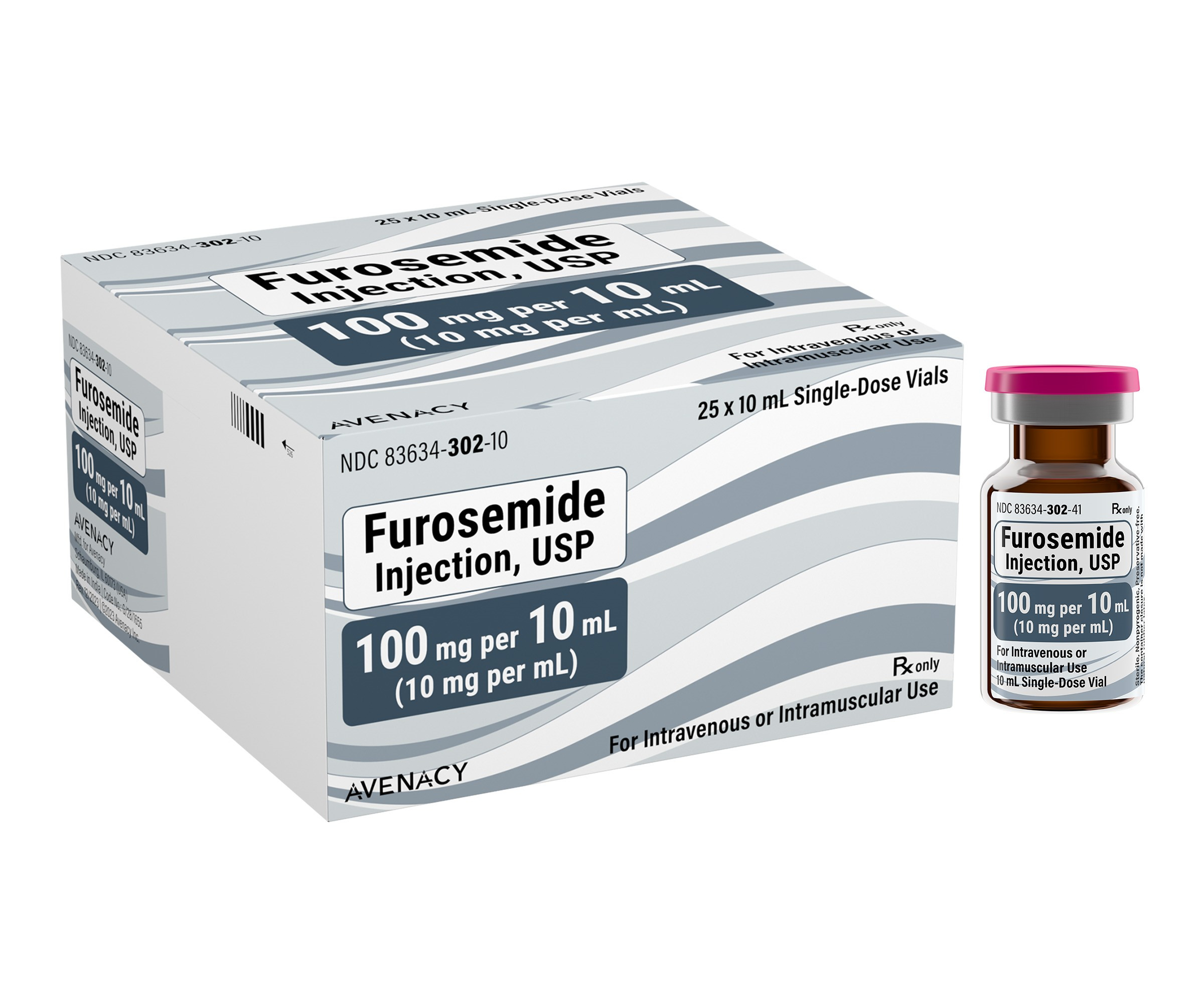
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Furosemide for Injection in the United States as a therapeutic equivalent generic for Lasix® for Injection (furosemide) approved by the U.S. Food and Drug Administration. Furosemide is indicated in adults and pediatric patients for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome. Furosemide is particularly useful when an agent with greater diuretic potential is desired.
By Avenacy · Via Business Wire · March 20, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Fosaprepitant for Injection and Fulvestrant Injection in the United States.
By Avenacy · Via Business Wire · March 18, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Bivalirudin for Injection in the United States as a therapeutic equivalent generic for Angiomax® for Injection (bivalirudin) approved by the U.S. Food and Drug Administration. Bivalirudin for Injection is indicated for use as an anticoagulant for use in patients undergoing percutaneous coronary intervention (PCI) including patients with heparin-induced thrombocytopenia and heparin-induced thrombocytopenia and thrombosis syndrome.
By Avenacy · Via Business Wire · January 29, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Melphalan Hydrochloride for Injection in the United States as a therapeutic equivalent generic for Alkeran® for Injection (melphalan hydrochloride) approved by the U.S. Food and Drug Administration. Melphalan Hydrochloride for Injection is indicated for the palliative treatment of patients with multiple myeloma for whom oral therapy is not appropriate.
By Avenacy · Via Business Wire · January 16, 2024

Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it will be attending the 2023 American Society of Health-System Pharmacists (ASHP) Midyear Clinical Meeting & Exhibition in Anaheim, California on December 3-7, 2023. At the conference, the Company will be showcasing its expanding portfolio of injectable medications, differentiated business model, and experienced leadership team, as well as hosting meetings with potential customers and partners. Interested parties can visit Avenacy at exhibit booth #929.
By Avenacy · Via Business Wire · November 27, 2023

Avenacy, a specialty pharmaceutical company focused on developing, manufacturing, and delivering critical medications, today announced its launch. Supported by a vast global network of development and manufacturing partners, Avenacy is prepared to meet the needs of today’s dynamic drug supply chain while ensuring quality of care to patients.
By Avenacy · Via Business Wire · October 23, 2023
