– Substantial and durable reductions in convulsive seizure frequency observed in patients treated with 2 or 3 doses of 70mg followed by 45mg maintenance dosing on top of the best available anti-seizure medicines –
– Patients experienced continuous improvements in multiple measures of cognition and behavior with ongoing treatment through 2 years –
– Data support proposed Phase 3 registrational study regimen; Update anticipated before year-end –
– Zorevunersen generally well-tolerated across the studies –
– Data to be presented at the American Epilepsy Society (AES) 2024 Annual Meeting; Company to host virtual event for investors and analysts on Monday, December 9 at 8:30 am Eastern (5:30 am Pacific) –
Stoke Therapeutics, Inc. (Nasdaq: STOK), a biotechnology company dedicated to restoring protein expression by harnessing the body’s potential with RNA medicine, today announced new data from an analysis of nine patients treated with an initial 2 or 3 doses of 70mg, followed by 45mg maintenance dosing in the Phase 1/2a and open-label extension (OLE) studies of zorevunersen. Substantial and durable reductions in convulsive seizure frequency were observed in these patients who received zorevunersen on top of the best available anti-seizure medicines. In addition, patients treated in the OLE studies showed continuous improvements in multiple measures of cognition and behavior with ongoing treatment through 2 years. Zorevunersen was generally well tolerated across the studies. Together, these data support the company’s proposed Phase 3 registrational study regimen and its efforts to develop zorevunersen as a disease-modifying medicine for the treatment of Dravet syndrome.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241206101127/en/
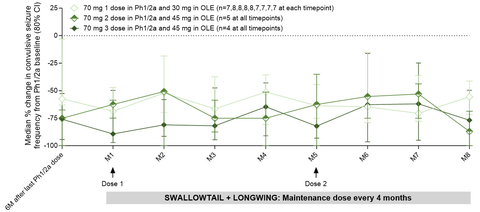
Figure 1. Substantial and Durable Reductions in Convulsive Seizure Frequency from Baseline in Patients who Received 70mg zorevunersen in Phase 1/2a (Graphic: Business Wire)
The company will host a virtual educational event for investors and research analysts on Monday, December 9 at 8:30 am Eastern (5:30 am Pacific).
“Dravet syndrome is stressful to live with and challenging to treat. Anti-seizure medicines are helpful in reducing seizures but can only do so much for patients who continue to experience significant and life-altering limitations in many aspects of neurodevelopment and daily living,” said Joseph Sullivan, M.D., FAES, Professor of Neurology and Pediatrics and Director of the Pediatric Epilepsy Center of Excellence at the University of California San Francisco. “The substantial and durable reductions in seizures, as well as the continuous gains in multiple measures of behavior and cognition through 2 years in patients treated in these studies have never been seen before in studies of Dravet syndrome. I am encouraged by these data and convinced that we are on the verge of a new era in the treatment of this disease.”
“The new long-term data give us a more complete and convincing picture of the potential for zorevunersen as a disease-modifying medicine,” said Barry Ticho, M.D., Ph.D., Chief Medical Officer of Stoke Therapeutics. “Patients entered our studies with high seizure rates despite being treated with the best available anti-seizure medicines. The durability of the substantial reductions in their seizures, particularly among those treated with initial doses of 70mg are remarkable and support a highly differentiated mechanism of action for zorevunersen. These data, combined with the continuous improvements in cognition and behavior, are highly supportive of our plans to conduct a Phase 3 study and the dose regimen currently under discussion with global regulatory agencies.”
Key Study Finding: Substantial and Durable Reductions in Convulsive Seizure Frequency
Poster 2.379 and 2.364
Previously reported end-of-Phase 1/2a study data from patients treated with two or three doses of 70mg of zorevunersen showed substantial and sustained reductions in convulsive seizure frequency of 85% at 3 months (n=10) and 74% at six months (n=9) post-last dose.
The Company is now reporting data for the nine patients who continued treatment with at least two doses of 45mg of zorevunersen in the OLE study. These patients sustained at least a 50% median reduction from baseline at each month of the OLE and demonstrated an 87% median reduction at month eight, the latest timepoint for which data have been assessed for these nine patients. See Figure 1.
Key Study Finding: Continuous Improvements in Multiple Measures of Cognition and Behavior
Patients experienced continuous improvements in multiple measures of cognition and behavior as measured by the Vineland-3 through 2 years of treatment with ongoing maintenance dosing in the OLEs. Additional improvements were indicated within the first nine months of treatment among patients in the Phase 1/2a ADMIRAL study. See Figure 2.
Key Safety Findings
At the time of the analysis, 81 patients had been treated with zorevunersen in the Phase 1/2a studies. Seventy-four patients who completed the Phase 1/2a studies and were eligible enrolled in the OLEs. As of June 2024, 82% (61/74) remained in the OLE studies. Safety findings from the studies are consistent with prior disclosures and are summarized below.
- Zorevunersen was generally well-tolerated across the Phase 1/2a and OLE studies.
-
In the Phase 1/2a studies:
- 30% (24/81) of patients experienced a treatment-emergent adverse event (TEAE) that was related to study drug. The most common were CSF protein elevations and procedural vomiting; and
- 22% (18/81) of patients had a treatment-emergent serious adverse event. These events were assessed as unrelated to study drug except for the previously reported case of one patient who experienced Suspected Unexpected Serious Adverse Reactions (SUSARs).
- A greater incidence of CSF protein elevation was observed in the OLEs. 79% (56/71) of patients in the OLEs had at least 1 CSF protein value >50 mg/dL. No clinical manifestations have been observed in these patients.
- Across the studies, one patient discontinued treatment due to study drug. As previously reported, this patient discontinued treatment in the OLE due to elevated CSF protein.
Additional Presentations
Small Changes on the Vineland-3 are Meaningful to Caregivers of Patients with Dravet Syndrome
Poster: 3.383
The Company will also present the results of a study of caregivers and clinical experts that was designed to evaluate what constitutes meaningful change on the Vineland-3 and to generate insight into caregiver perceptions of the key signs, symptoms and impacts of Dravet syndrome. The study found that small changes in adaptive behavior, as measured by the Vineland-3, are considered clinically meaningful by both caregivers of patients with Dravet syndrome and clinical experts. Changes of 2 to 3 points in growth scale values across subdomains were considered meaningful by at least 50% of caregivers. Consistent with other publications, the Expressive Communication and Receptive Communication subdomains were ranked by caregivers as the most important areas to improve with treatment. The study further defines meaningful change thresholds on the Vineland-3 scale to explore cognitive and behavioral benefits in clinical trials of potential disease-modifying treatments.
Spectral EEG Analysis Demonstrates Decreased Slow-wave Activity in Patients with Dravet Syndrome after Treatment with Zorevunersen, an Antisense Oligonucleotide
Poster: 3.407
An EEG analysis of 74 patients treated in clinical studies of zorevunersen showed a reduction in slow-wave activity following zorevunersen administration. The treatment effect on spectral power is most pronounced at the highest zorevunersen doses, with reductions in slow-wave activity sustained for months after the last dose. Topographical spectral changes indicate that zorevunersen produces a widespread and durable effect across the brain.
All presentations are available for download on the Stoke Therapeutics website under the Investors & News tab.
Stoke Therapeutics Analyst and Investor Virtual Event
Stoke will host a virtual event with discussions led by leading clinicians and patient advocates to offer analysts and investors an opportunity to learn more about Dravet syndrome, its overall effects, and the potential real-world impacts of a disease-modifying medicine. As part of this event, the clinicians are expecting to share anecdotes and visuals from their experience treating patients in the clinical studies of zorevunersen.
Title: Understanding Dravet Syndrome: The Unmet Need and Potential for Disease-Modification
Date and Time: Monday, December 9, 8:30-9:30 AM EST (5:30-6:30 AM PST)
Presenters: Edward M. Kaye, M.D., CEO of Stoke Therapeutics, Joseph Sullivan, M.D., FAES, Professor of Neurology and Pediatrics and Director of the Pediatric Epilepsy Center of Excellence at the University of California San Francisco; Andreas Brunklaus M.D., Consultant Paediatric Neurologist at the Royal Hospital for Children, Glasgow, Honorary Professor at the University of Glasgow, member of Dravet Syndrome UK's Medical Advisory Board; Mary Anne Meskis, Executive Director, Dravet Syndrome Foundation; and Veronica Hood, PhD, Scientific Director, Dravet Syndrome Foundation
Webcast Link: https://edge.media-server.com/mmc/p/bv6h2oxs
About Dravet Syndrome
Dravet syndrome is a severe and progressive genetic epilepsy characterized by frequent, prolonged and refractory seizures, beginning within the first year of life. Dravet syndrome is difficult to treat and has a poor long-term prognosis. Complications of the disease often contribute to a poor quality of life for patients and their caregivers. The effects of the disease go beyond seizures and often include intellectual disability, developmental delays, movement and balance issues, language and speech disturbances, growth defects, sleep abnormalities, disruptions of the autonomic nervous system and mood disorders. The disease is classified as a developmental and epileptic encephalopathy due to the developmental delays and cognitive impairment associated with the disease. Compared with the general epilepsy population, people living with Dravet syndrome have a higher risk of sudden unexpected death in epilepsy, or SUDEP. There are no approved disease-modifying therapies for people living with Dravet syndrome. One out of 16,000 babies are born with Dravet syndrome, which is not concentrated in a particular geographic area or ethnic group.
About Zorevunersen
Zorevunersen is an investigational new medicine for the treatment of Dravet syndrome currently being evaluated in ongoing clinical trials. Stoke believes that zorevunersen, a proprietary antisense oligonucleotide (ASO), has the potential to be the first disease-modifying therapy to address the genetic cause of Dravet syndrome. Zorevunersen is designed to upregulate NaV1.1 protein expression by leveraging the non-mutant (wild-type) copy of the SCN1A gene to restore physiological NaV1.1 levels, thereby reducing both occurrence of seizures and significant non-seizure comorbidities. Zorevunersen has been granted orphan drug designation by the FDA and the EMA. The FDA has also granted zorevunersen rare pediatric disease designation and Breakthrough Therapy Designation for the treatment of Dravet syndrome with a confirmed mutation, not associated with gain-of-function, in the SCN1A gene.
About Stoke Therapeutics
Stoke Therapeutics (Nasdaq: STOK), is a biotechnology company dedicated to restoring protein expression by harnessing the body’s potential with RNA medicine. Using Stoke’s proprietary TANGO (Targeted Augmentation of Nuclear Gene Output) approach, Stoke is developing antisense oligonucleotides (ASOs) to selectively restore protein levels. Stoke’s first compound, zorevunersen (STK-001), is in clinical testing for the treatment of Dravet syndrome, a severe and progressive genetic epilepsy. Dravet syndrome is one of many diseases caused by a haploinsufficiency, in which a loss of ~50% of normal protein levels leads to disease. Stoke is pursuing the development of STK-002 for the treatment of autosomal dominant optic atrophy (ADOA), the most common inherited optic nerve disorder. Stoke’s initial focus is haploinsufficiencies and diseases of the central nervous system and the eye, although proof of concept has been demonstrated in other organs, tissues, and systems, supporting its belief in the broad potential for its proprietary approach. Stoke is headquartered in Bedford, Massachusetts with offices in Cambridge, Massachusetts. For more information, visit https://www.stoketherapeutics.com/.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955, including, but not limited to: the ability of zorevunersen (STK-001) to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in behavior or cognition at the indicated dosing levels or at all; the timing and expected progress of clinical trials, data readouts, regulatory decisions and other presentations for zorevunersen, including the timing and presentation of data at AES 2024; the potential for zorevunersen to be the first disease-modifying therapy for Dravet syndrome; the timing of regulatory interactions or the outcomes thereof; our expectations, plans, aspirations and goals, including those related to the potential of zorevunersen. Statements including words such as "anticipate," "believe," "hope," "plan," "will," "continue," expect," "ongoing," or "potential" and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties related to: our ability to advance, obtain regulatory approval of, and ultimately commercialize our product candidates, including zorevunersen; the timing of data readouts and interim and final results of nonclinical and clinical trials; nonclinical and clinical data are voluminous and detailed, and regulatory authorities may interpret or weigh the importance of data differently and reach different conclusions than us or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; receiving Breakthrough Therapy Designation may not lead to a faster development or regulatory review or approval and does not mean zorevunersen will receive marketing approval; our ability to fund development activities and achieve development goals; our ability to protect our intellectual property; global business, political and macroeconomic conditions, including inflation, interest rate volatility, cybersecurity events, uncertainty with respect to the federal budget, instability in the global banking system and volatile market conditions, and global events, including public health crises and ongoing geopolitical conflicts, such as the conflicts in Ukraine and the Middle East; and other risks and uncertainties described under the heading "Risk Factors" in our Annual Report on Form 10-K for the year ended December 31, 2023, our quarterly reports on Form 10-Q and the other documentation we file from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241206101127/en/
Contacts
Stoke Media & Investor Contacts:
Dawn Kalmar
Chief Communications Officer
dkalmar@stoketherapeutics.com
781-303-8302
Doug Snow
Director, Communications & Investor Relations
IR@stoketherapeutics.com
508-642-6485
